Studies on Russian Economic Development, Vol. 10, No. 2, 1999, pp. 199-211.
Original Russian Text Copyright © 1999 by Zakharov.
English Translation Copyright © 1999 by MAHK "Hayêa/lnterperiodica" (Russia).
Abstract-Methodological and pragmatic aspects of the study of disparities between male and female mortality in post-WWII Russia are discussed in three dimensions-age, birth cohort, and calendar time. The author's analysis bears out the hypothesis of a considerable variation in mortality between birth cohorts (generations by year of birth) in Russia.
General statement of the problem and a brief historical background. Russia was one of the last among advanced European nations to embark on the course of an epidemiological transition associated with a decline in mortality and a profound change in of the age and cause patterns of mortality. When it started its gradual progress toward higher longevity in the late 19th-eariy 20th century, Russia had been lagging behind the West by half a century or more. Its lag behind Central and Eastern European countries, though smaller, was substantial, too. Russia's starting positions with respect to mortality rate were far less favorable than those of any advanced country prior to the shift. The main characteristics of Russian mortality were extremely high child, especially infant, mortality (more than 300 infant deaths per thousand births), and also a particularly adverse situation with infectious and parasitic diseases, including fatally dangerous ones like cholera, typhus, diphtheria, scarlet fever, measles, and pox. Although tuberculosis ranked among the chief causes of mortality at the time, Russia did not stand out among the other European countries by a particularly high rate of mortality from tuberculosis1.
Russia's progress by way of the epidemiological transition from the early 20th century to the mid-1990s differed from that of the other advanced nations, primarily because of a long chain of social disasters which befell the Russian population in the first part of this century2. As a result, Russia's mortality dynamics followed a curvy path dotted with huge leaps in the periods of socioeconomic upheavals. Certain gains in the control of infectional pathology achieved in its quieter years were nullified by frequent crises of social ethiology which led to new cycles of rising child mortality due to violence, starvation, malnutrition, and outbreaks of epidemics. Among the developed nations in the 20th century, Russia appears to lead in absolute and relative demographic losses suffered on account of wars, revolutions, famines, epidemics, and the state's mass violence towards its own people. This naturally raises the question of how these mass and chronic deprivations have affected the viability of generations which have lived through catastrophies. Is there excess mortality in cohorts which find themselves in the least favorable conditions at some point in their lifetime?
World literature has accumulated enough evidence to suggest that the living conditions under which child bearing, and the child's physical development and socialization take place have a decisive influence on their resistivity to diseases and death in adult life.3 Diseases for which there are multiple proven clinical and demographic correlations with childhood living conditions are tuberculosis of the lungs, respiratory infections, bronchites, hepatitis B, cirrosis and cancer of the liver, rheumatic and ischemic diseases of the heart, and all cardiovascular pathology, for that matter.4
In recent years, researchers have given much attention to the human anthropometric indicator of body height, which is currently regarded as the best indicator of early-life living conditions and physical development. Studies were made, which have since become widely popular, to suggest an inverse and surprisingly strong relation between a person's height and his/her probability of dying from cardiovascular diseases5. The relation remains strong even if traditional behavior-specific risk factors are controlled, such as smoking, food habits, blood pressure, as well as those not directly related to a person's behavior, e.g., educational background, occupation, place of residence, body mass index, and possible pathologies in case histories like tonsillitis, diabetes, high cholesterol level, etc. Researchers have thus acquired an indicator which has a great explicative force and can, in addition, bring a historical context into the study of mortality differentials factors. The problem of the prevalence of short stature in Russia's adult population, which is commonly associated with low resistivity to diseases and higher mortality, has drawn the attention of students of population health in Russia, notably under a well-known longitudinal study, RLMS (the project leader is B. Popkin of the University of North Carolina, USA).
Relative to Russia the study of birth cohort mortality differentials has a comparatively recent history. Apart from some observations on higher mortality among generations born during WWI, the Civil War in Russia, and WWII, which were made by Soviet researchers (M.S. Bednyi,6 V.A. Minyaev and I.V. Polyakov,7 in the 1960s-1970s, it was not until the late 1980s that the question received serious attention. Of course, it was as late as 1987 that age-specific mortality data were made public in this country. At that time a tool known as APC (Age-Period-Cohort) analysis had been gaining worldwide popularity. For a particular hypothesis it permits to separate the general mortality variation over a period into constituents depending on the factors of age, calendar period, and membership in birth cohorts.8
At first it was Anderson and Silver 9, followed by Willekens, Scherbov, and Andreev 10 who applied APC analysis in various modifications to mortality data of the USSR, to include individual Union Republics and causes of death (five-year age-specific death rates from 1958/1959 to the late 1980s). Later on the technique of APC analysis was applied to more detailed data covering a longer period in Russia (V.M. Shkolnikov and S.V. Adamets) and Ukraine (E. Godek and V.M. Shkolnikov).11
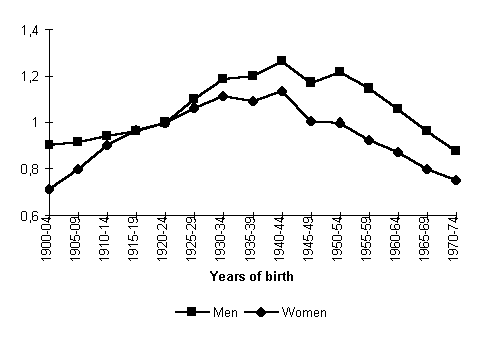
Figures 1-3 give a graphic form to the findings of an APC analysis relative to the total population of the former USSR computed by Willekens and Scherbov.12
It is noteworthy that both the age, period, and cohort effects are higher in male mortality than in female mortality. It goes to show once again that men have the higher specific vulnerability no matter how relative the measurement: by age, calendar year, or year of birth. Even though they used somewhat differing calculation techniques and arrived at correspondingly varying numeric estimates of age, period, and cohort effects, the above authors came to similar conclusions:
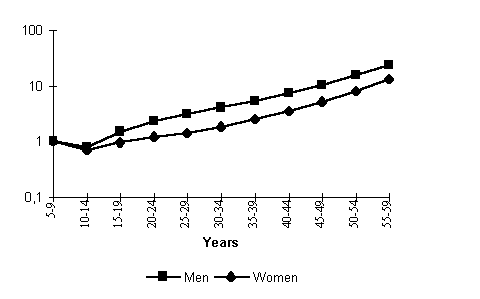
Without going into the unique features of the APC analysis tool and its merits and demerits, we shall merely observe that by its very nature it cannot take into consideration a highly important characteristic of the Russian history of mortality which has a direct bearing upon something referred to as part of mortality variation accountable to cohort effect. To clarify, Russia's population has lived in the 20th century through a long period of all-round deprivation: a very low standard of living over decades, three wars, three famine periods, state-orchestrated mass violence against particular social and ethnic groups, often acquiring genocidal proportions, and millions kept in prison camps. The worst deprivations, now harder, now lighter, fell to the lot of generations born within the interval around 1915 through 1955. The APC analysis fails to do justice to this characteristic and may interpret the remote effects of a prolonged intermittent deterioration of past living conditions as a permanent or periodic deterioration of present living conditions, nearer to the observer. Because of that, chances are that the factor of historical conditions, which influences current mortality, will be underestimated. In addition, as noted by Willekens and Scherbov,13 APC analysis cannot separate correctly period effect and cohort effect where present living conditions elicit different mortality responses from different cohorts; this is particularly true of the case when mortality data apply to five-year or even larger general age groupings. It will be demonstrated that originally weakened cohorts produce sharper responses to changed conditions than do more fortunate cohorts; at least this has been so in Russia.
Another important question that needs to be clarified is the length of time for which we should interpret variations in death rates in terms of the possible effect of current conditions and the contribution of cohorts with initially different survival functions. Techniques like APC analysis which rely on regression equations, or more accurately, general linear models, require long dynamic series of age-specific death rates. The dimensionality of the death rate matrix (age x observation years) must be sufficient for stable assessment of the model parameters. Of course, any discovered effects which are attributed to age, period, and cohorts are mean values referring to the entire, fairly long observation period. These methods prove to be largely helpless when applied to the study of the kind of short, wave-like surges in mortality that Russia experienced in the 1980s-1990s.
One final observation is that without reference to the data analysis technique, i.e., considering the most general case of observation of death rate variation over a fixed time period, the problem of separation of the effect of long-term factors from medium- and short-term ones has no single solution without recourse to extra information. Researchers were aware of this long before the advent of computer processing and sophisticated mathematical representations of data. After all, data used have changed little since that time, either in substance or information content, least of all in Russia.
We demonstrated in earlier studies, without recourse to the APC analysis, disparities in mortality between Russian cohorts 14. Disparities in mortality between persons grouped by year of birth prove to be quite marked when we compare figures for the same age groupings between neighboring "more fortunate" and "less fortunate" cohorts. To satisfy ourselves of the truth of this statement we may refer to Russia's numbers of the deceased in the postwar period grouped by one-year age groupings. Regrettably, in this country death statistics have not been maintained simultaneously by age and year of birth, which tends to complicate our calculations and somewhat lowers their accuracy in the sense of assigning demographic events to one age cohort or another.
The inequality of birth cohorts in Russia in terms of mortality and longevity is no longer a hypothesis but a proven fact. Likewise we accept it as proven that there is cohort inequality in France, Italy, Germany, Poland, and Japan 15. It is important that this fact be described in every detail, illustrated by examples, showing in particular how it manifests itself in the different periods of cohorts lifetime and different calendar time periods, and its implications for the general mortality dynamics.
Characteristics of source data. As mentioned previously, modem Russian vital statistics, like Soviet and prerevolutionary Russian statistics before it, have no suitable mechanism for registration of demographic events to allow the representation of data for parallel cohort, age, and momentary analysis in the spirit of the well-known Lexis diagram. What is available is merely annual distributions of the numbers of the deceased by one-year age group, but not simultaneously by year of birth and age, as required by the ideal scheme. A more or less reliable series of age distributions of deaths for Russia that we have at the moment refers to all the years starting with 1959.
Therefore, passing from an age distribution to a cohort distribution requires the use of some approximation procedures, which cannot transform ideally one summary actual number of the deceased in each age into two unknown numbers of the deceased belonging to two neighboring birth cohorts. The problem is even more complicated in the case of sharp year-to-year changes in living conditions and correspondingly sharp fluctuations in numbers of the births and deaths in response to these changes. Any approximation, however sophisticated, is bound to lead to some evening out of the effect of disparities in mortality between successive cohorts.
Another problem to be reckoned with is the heaping of the numbers of the deceased of specific ages and years of birth, due to mistakes in reporting a deceased/born person a particular age/year of birth. Without going into the causes of this rather complicated phenomenon, we shall merely observe that heaping in Russia reveals itself clearly in ages ending in 0 or 5, and partly so in ages 12 and 16. Note that the age heaping of both the deceased and the survivors at the census in Russia visibly decreased in the 1960s-1980s as the share of persons of advanced age grew who were sure about their date of birth and who had received a passport giving their age by their birth certificate. Age heaping increased somewhat in the 1990s, probably on account of numeric growth of the deceased whose identity and/or time of death could not be ascertained with certainty. In addition to heaping age there is quasi-heaping whereby a person will be classified under a specific year of birth (might it not be in consequence of people having ascribed to themselves "desirable" years when the national passport issuance campaign began?) Thus, years ending in 0 in the 19th century, especially the year 1900, were popular as years of birth.
It appears that no formal procedures are available which would help us eliminate man-made heaping completely and to arrive at the actual age (cohort) distribution of the numbers of the living and the deceased, especially in relation to Russian data, where the age pattern at any given moment is disturbed by the interference of real high-frequency demographic waves produced by fluctuations in the number of past births and man-made fluctuations caused by misreporting. The most we can do is minimize, by using near-heuristic methods, the incidence of cases when the above-mentioned waves are in resonance, producing fluctuations of an incredibly high amplitude. In addition, our work experience suggests that it is not advisable to smooth out the numbers of deaths by the age scale before these data have been transformed into cohort form and mortality indicators (mortality probabilities) have been obtained. It is in cohort presentation that mistakes in age reporting manifest themselves most strikingly, with the exception of cases of systematic assignment of the wrong year of birth to people, which are by far less pronounced compared to heaping proper.
However, no matter how serious the problem of integrity of Russian data would appear from the statistical angle, it is minimal in comparison to the problem of the shortness of our period of continuous observation.
Cohort studies of mortality, in view of the average human span of life, depend on continuous data series for a long period, preferably 100 years or more. What we have at our disposal, though, is Russian mortality data for a period of less than 50 years. We lack reliable information about child mortality for all pre-1959 cohorts 16. Lengths of calendar time for which information is available often prove to be too short to permit us to compare the mortality levels of a sufficiently long series of cohorts as they reach different ages, from youth to old age. To make it plain, it turns out that for one, rather short series of cohorts we can assess disparities in young ages alone, for another series, in middle ages, and for a third, in old age. (See Tables 1 and 2 and Appendices.)
Obviously, to lift this limitation we must extend our information file to a more distant perspective. Theoretically, it can be done. Russian archives contain necessary data in suitable formats covering a sufficiently long period of time, at least since the late 1920s. However, the practical utilization of this information runs up against numerous difficulties in assessing the completeness of available data, among them the considerable underreporting of deaths, a variety of systems for control of mortality of civilian population and special contingents, and the impermanence of referencing of the numbers of events to territorial divisions. As we go back farther in the past the above difficulties turn into daunting problems. We can only hope that as historical and population studies are expanded the problem of data scarcity will gradually diminish. We can hardly expect its early solution, though.
Study results. We separated the total observation period into two unequal lengths: a 25-year period of slow evolutionary changes in mortality until the state's conscious interference with a view to improving the situation (in fact, until the anti-alcohol campaign) and a 10-year period following the interference which coincided with sweeping socioeconomic changes in Russia.
Inequality of generations in the face of death in 1959-1984. Tables 1 and 2 list the mortality probabilities for all male and female cohorts born from 1900 to 1960 for aggregated age intervals (q20-30, q30-40, q40-50, q50-60, q60-70) and for the total observation period, 1959 to 1995. (See Appendix.)17
Note that besides the above-mentioned generations, those born in the 1950s have a higher death risk, too. This question calls for a special investigation, all the more so as this phenomenon has been found not only by us on Russian data, but by other students of cohort disparities in mortality, e.g., in France 18. On the one hand, these birth cohorts in Russia were the first to be exposed to new methods of treatment, notably antibiotics, which might have had dual remote effects: (a) generations became more heterogeneous owing to the survival of weakened children, which provoked high mortality at a more mature age, and (b) the application of new drugs had more remote aftereffects. On the other hand, the age of antibiotics came to France a decade and a half before Russia. Specialists offer a different explanation for France, namely, that it was in that period that the nation carried out a changeover to centralized, hospital-based modes of child delivery, which might have had a dual, contradictory effect-a generally positive effect for early life periods and a negative delayed effect upon the average survivorship of generations less homogeneous in terms of viability.
Another hypothesis may be advanced which no doubt must be verified but whose logic seems to explain the similarity of the cohort effect found in different countries. The cohorts born in the 1950s are children of those who were born during the grim period of the late 1920s or early 1930s. In the West it was a terrible economic crisis, and in Russia, the even more dreadful collectivization and famine. The fact that the 1920s-1930s cohorts in many countries display higher mortality suggests that the historical circumstances of their birth were very unfavorable.
The hypothesis that less viable cohorts bring forth less viable progeny is not only logical in itself but has a number of empirical arguments to support it. Thus, a study of the consequences of the famine winter of 1944-1945 in Holland, which rested on special-purpose cohort groupings of the full registers of those born in that period, revealed not only a higher mortality in these birth cohorts, but also an elevated mortality of their children, «grandchildren of war».19 In Russia, too, we have found higher mortality in the children of mothers born in the WWII period; our source data were different, however: the numbers of ever born and surviving children by the one-year age group of their mothers.20 Thus, by our estimates, the children of mothers born in the hardest years, 1942-1943, had 5-10% higher mortality than of those born in the final postwar years. A similar analysis of the remote consequences of the famine of the 1930s is impossible in view of the absence of equally detailed data for the cohorts of mothers who were born in those years.
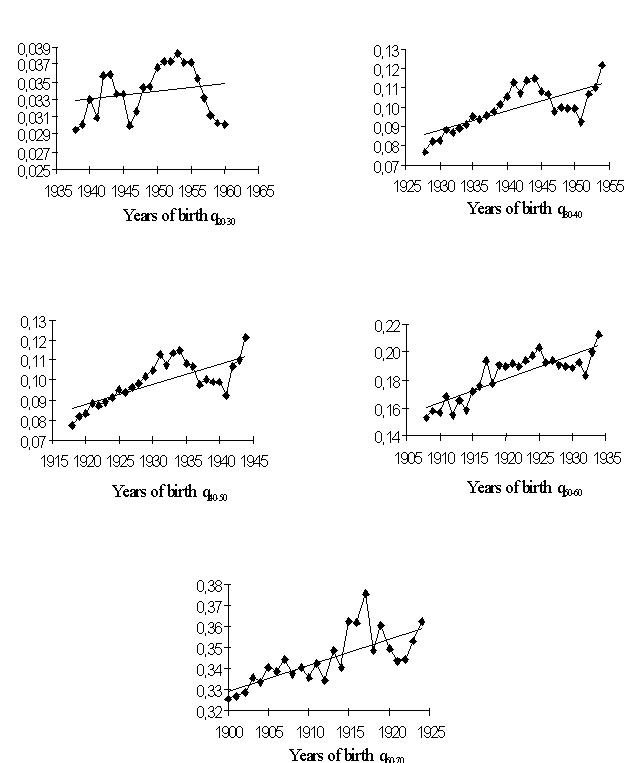
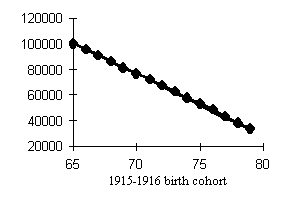
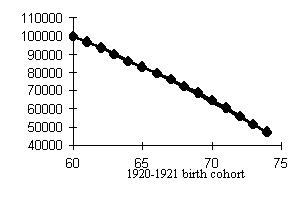
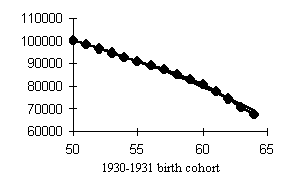
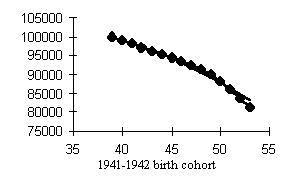
Data of Tables 1 and 2 help assess the higher mortality levels in the least fortunate cohorts. Let us compare cohorts having peak mortality levels with those bom in more favorable years. If we dwell on males (Fig. 4), the mortality of, e.g., the 1917 cohort was 15.3% higher within the 50-60 age interval and 9.7% higher within the 60-70 age interval than that of the 1911 cohort. The mortality of the 1934 cohort was 16.9% higher within the 30-40 age interval and 26.8% higher within the 40-50 age interval than that of the, 1928 cohort. Those born in 1942 exhibited a mortality rate 20.8% higher within the 20-30 age interval and 23.4% higher within the 30-40 age interval than did the 1938 cohort.
Female cohorts have a similar pattern; their variations in mortality are generally smaller, still quite pronounced. The 1917 cohort had mortality 13.2% higher in the 50-60 age interval and 10.9% higher in the 60-70 age interval than did the 1911 cohort. The mortality of the 1934 cohort in the 30-40 age interval was 6.3% higher and in the 40-50 age interval 14.1% higher than that of the 1928 cohort. The 1942 cohort showed a mortality rate 5.0% higher in the 20-30 age interval and 22.6% higher in the 30-40 age interval than did the 1938 cohort.
Our analysis of the cohort dynamics of mortality has led us to an important and, alas, sad conclusion. Mortality shows a visible upward trend which cannot but affect the survivorship levels of cohorts starting with the 1915-1916 birth cohorts up to the cohorts born during WWII in men and women, the former worse off than the latter. Besides, while the postwar generations of women demonstrate a distinct tendency toward lower mortality in younger ages, men so far have not showen any hopeful signs with the exception of advances in reducing child mortality. Thus, the probability of dying at ages 30-40 is 1.5-2 times higher in the male cohorts born in the early 1950s than in those born in the 1920s, i.e., those who fought in WWII!
It remains to be analyzed which of the factors was crucial here: was it the extremely weakened health of the cohorts born in the first post-WWII decade; these generations' flagrantly inadequate vital behavior, ill-disposed to good health; or a peculiar selective effect of the pre- and postwar years which led to the survival of the strongest in the cohorts born in the 1920s.21 One would hardly contend that the general socioeconomic conditions, health care, and living conditions of the Russians were worse in the late 1970s-early 1980s than they were in the early 1950s; but these are precisely the periods to be compared when we contrast age-specific mortality rates for the above cohorts. Anyhow, it is quite obvious that Soviet society proved to be unable to withstand such powerful negative trends in population health, with the result that a "children's" generation has a higher mortality, at the same age, than their "parent" generations.
In contrast to other developed countries for which cohort-specific data are available, Russia's "unfortunate" cohorts demonstrate higher mortality against the background of a lasting negative trend. In Western countries, "problem" cohorts will stand out by virtue of a short-term rise in mortality or merely some slowing down of its decrease, this against the backdrop of a general positive trend which indicates the lowering of mortality from cohort to cohort. This goes to show that the boons of civilization can, in principle, offset much of the ill health inherited at birth or acquired in childhood, thereby smoothing out the inequality of people who, though belonging to different cohorts, are only a few years apart in age. In the 20th century Russia, on the other hand, whether because disparities in the conditions of birth and subsequent life between generations proved too great, or because generations of sickly parents brought forth sickly progeny, or because again the general progress of the nation in the Soviet period was not, contrary to official declarations, geared to provide chances of survival to the weaker citizens-it is an undisputable fact that in Russia the inequality in mortality between demographic generations is extremely high. This inequality manifested itself in the peaceful postwar years, long before the socioeconomic crisis of the 1980s-1990s and the present turmoils connected with societal change.
If we examine long-term mortality trends from a cohort perspective we shall be able to update the common periodization of mortality dynamics in Russia. Thus, it is widely believed that the current epidemiological crisis started in the mid-1960s. A cohort analysis will testify, however, that negative tendencies in middle- and old-age mortality have a longer history and can be traced as far back as 1959. In all probability, these tendencies appeared even earlier.
Dissimilar responses of generations in a period of sharp variation in mortality: the mid-1980s to mid-1990s. During that decade Russian society lived through great social upheavals. First, the recognition of the need for radical reform and early steps towards a democratic society, followed by a sudden start and erratic conduct of reforms in all walks of social life, affecting all aspects of people's day-to-day activities-all this became intermingled in a fast-moving succession of events and developments, among which one is hard put to isolate key factors relevant to population mortality dynamics. Meanwhile, Russia has experienced, since the mid-1980s, a unique surge in mortality, unprecedented among civilized nations, which has been drawing the attention of specialists, politicians, and media the world over. Suffice it to say that the mortality level for individual age groups in Russia has varied not by percents-nothing unusual in other countries under regular circumstances-but by dozens of percents. Thanks to focused efforts of a number of international teams, including Russian professionals, we have learned much about the inner workings of these remarkable changes 22. Therefore we shall not examine a succession of sharp fluctuations in mortality-a decline in 1985-1987, a rise in 1988-1994, and another decline since 1995 to the present-nor shall we go into the details of hypotheses that have been put forward to explain this unique phenomenon, the more so, that both the hypotheses and their critique are described in the literature. We shall only dwell on those aspects of the facts which are relevant to our subject.
Members of different birth cohorts, as they grow older and live through the same ages, have different death probabilities. Mortality in Russia is not only age-heterogeneous, it is cohort-heterogeneous. When a series of cohorts pass through the same ages, which have different destinies and correspondingly different susceptibility to illness and death, this is reflected in death rate variations which, other conditions being equal, may not be connected with current alterations in the environment. When, however, the situation in the surrounding world for some reason suddenly changes for better or worse, from the angle of mortality, different cohorts with their specific resistivity may respond differently to environmental change by either enhancing or lowering their age-specific death selectivity. This line of reasoning is by way of introducition a somewhat unusual, cohort-based, approach to the study of the phenomenon of sharp variations in mortality in the last decade in Russia. In what ways has the inequality of generations, in the sense of disparities in susceptibility to the risk of death, manifested itself during sudden societal change?
Most analysts by now are quite positive that the anti-alcoholic campaign was the primary reason for the sharp reduction in high mortality in 1985-1987, when the life expectancy (LE) for the male newborn grew by more than four years, from 61.7 in 1984 to 65.0 in 1987, and for the female newborn, by one-and-a-half years, from 73.0 to 74.6 years. Further research 23 demonstrated that it was the post-1991 about-face in the alcohol market which triggered a sharp rise in mortality in 1992-1994, with male LE falling to 57.4 years and female LE to 71.0 years.
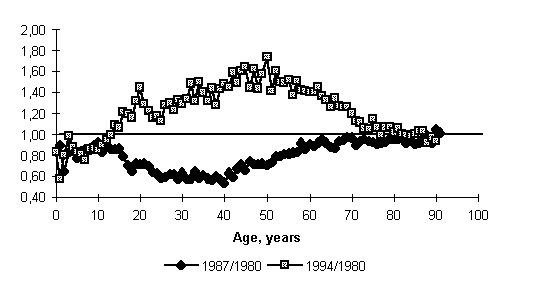
Now, let us turn to two distinct periods-a decline in mortality in the mid-1980s and a growth in the early 1990s-and compare the variation in age-specific mortality rates at two comer points, 1987 and 1994, relative to those in the base year, 1980. The reason for choosing 1980 as the base of comparison is that the mortality rate and its age profile at that particular point in time were undoubtedly the result of development of natural long-term trends, unaffected by the government's purposeful efforts to influence it.24
It is frequently stated in professional literature that a fall and a rise in mortality in the period of study fall on the same age groups. It is essentially correct if we consider aggregated age intervals such as children, middle-aged and able-bodied persons, old men, etc. However, if we look carefully at one-year age groups we shall see that a decline and a subsequent rise in mortality do not fall on exactly the same ages. The mortality increment in the 1990s shows a certain systematic bias to the right, towards the older ages, relative to the comparative decline of the 1980s (Fig. 5). It could be reasoned that the mortality crisis affected prepension age groups in the first place, and that would have led us to far-reaching socioeconomic conclusions about factors and implications in no way connected with the previous decline period. (It is a widely held view that persons of prepension age are perhaps the main victims of reform.) But are not such conclusions too hasty?
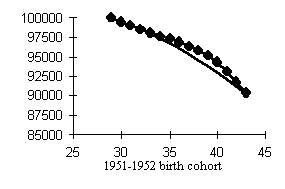
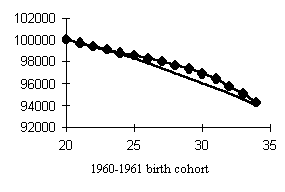
Let us consider the change in mortality figures over the same period, but in cohort rather than age representation, i.e., not on a relatively fixed age scale, but on a scale of cohorts years of birth (Fig. 6).
It will be seen that the curve becomes more symmetric, the relative decline and rise in mortality as if neutralizing each other. It turns out that the relative strength of response of the same cohorts during the mortality decline and rise periods had closely matching values but different signs. In other words, cohorts which gained the most from reduced mortality were the main losers somewhat later, and the other way round, cohorts which displayed the least response to change in the mortality decline period had an equally low response in the rise period. A symmetric effect was also observed in women, as well as in individual causes of death.
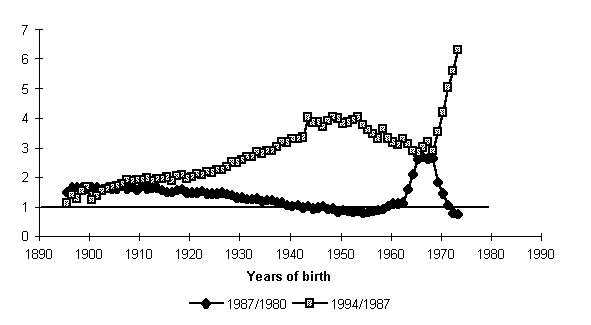
The symmetry axis separating the two curves relative to variations in Fig. 6 does not pass through unity and is generally non-linear relative to the years of birth of the cohorts marked on the X-axis. The explanation is simple enough-the age effect. Each cohort has become seven years older during each of the periods under review, and it is only natural that the death rates in cohorts varied not only owing to external factors related to a changed situation in Russia ("the period effect") but by virtue of cohort aging. What is intriguing is that for a number of cohorts born in the first postwar decade the positive effect of the antialcoholic campaign proved so strong as to actually overbalance the negative effect of aging of members of these cohorts. (In Fig. 6 the relative variation of their death rates is less than unity.) By their passports, people grew seven years older, and according to their mean death probability, more than seven years younger! Could a situation as unique as that last? Of course, not. A subsequent plummeting mortality was inevitable, if only because gerontology has not demonstrated its rejuvenation potential on a scale comparable to what had been achieved in the first two years after the launch of the antialcoholic campaign. Fig. 6 reminds us of another important demographic regularity which we shall do well to keep in mind when comparing the death rates' variation with time. Age effect, connected with the biological property of aging, invariably enhances the negative period effect tending to increase mortality, and vice versa, weakens the positive effect of favorable conditions when mortality declines.
For our further analysis we would do well to eliminate the factor of cohort aging, or age effect. It can be achieved by narrowing each observation period to one year. The increment of calendar years will then equal the increment of age in members of birth cohorts.
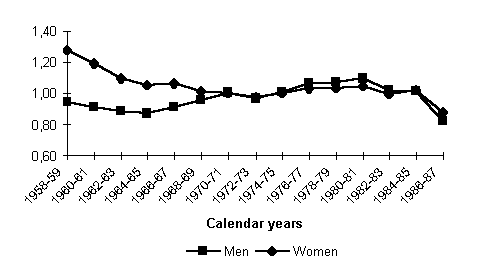
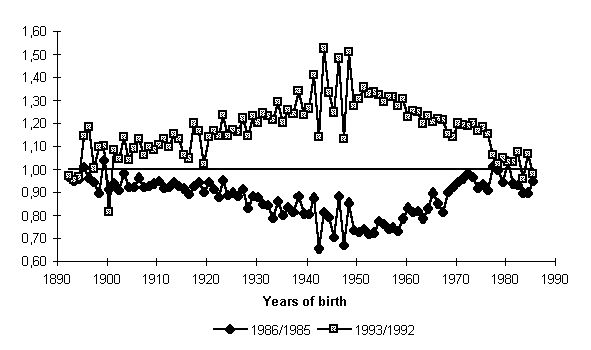
Figure 7 shows annual rates of decline and rise in mortality in one-year cohorts which were achieved in the peak years of decline and rise of total mortality, 1986 and 1993, respectively. Absolute symmetry in cohort mortality decline and growth is undisputable, even though the cohort dichotomy is somewhat higher in the 1990s.
This close match between cohorts in respect to mortality variation leads one to the hypothesis that:
 |  |
 |  |
 |  |
Let us ask ourselves, how would the survivorship of cohorts have changed if the age-specific mean death probabilities of the 1978-1980 had continued throughout the subsequent period. Let us then compare the predicted numbers of survivors and the accumulated numbers of deaths by cohort with available statistics. Our results for males look staggering even to a population scientist who has had enough experience with this kind of data.
Insignificant discrepancies between the actual and model survival functions which had appeared across all cohorts by 1996 is yet another confirmation of the main conclusion, namely, the almost perfect symmetry of two periods, a decline in mortality in the 1980s and its rise in the 1990s. Deviations from zero in isolated cohorts can easily be explained away by cohort selectivity in mortality or by the inequality of generations. The specific vulnerability of separate generations made itself more visible in a period of rapid societal change compared to a period of even, evolutionary change.
ACKNOWLEDGMENTS
This study was completed as part of two projects:
International Project for the Study of Mortality in the Adult Population of Russia in the 1980s-1990s (the research team included Russian, British, and French specialists, and the project was supported by Know-How Fund, Britain); and Social Inequality in the Face of Death in Russia (the project leader is V.M. Shkolnikov, the CDHE, Moscow, and the project was supported by Rockefeller Foundation, USA).
| Year of birth cohort | q 20-30 | q 30-40 | q 40-50 | q 50-60 | q 60-70 |
| 1900 | 0,32577 | ||||
| 1901 | 0,32701 | ||||
| 1902 | 0,3289 | ||||
| 1903 | 0,33546 | ||||
| 1904 | 0,33356 | ||||
| 1905 | 0,34041 | ||||
| 1906 | 0,33875 | ||||
| 1907 | 0,34428 | ||||
| 1908 | 0,15358 | 0,33769 | |||
| 1909 | 0,15820 | 0,34039 | |||
| 1910 | 0,15727 | 0,33537 | |||
| 1911 | 0,16797 | 0,34243 | |||
| 1912 | 0,15542 | 0,33426 | |||
| 1913 | 0,16509 | 0,34871 | |||
| 1914 | 0,15890 | 0,34063 | |||
| 1915 | 0,17222 | 0,36254 | |||
| 1916 | 0,17622 | 0,36172 | |||
| 1917 | 0,19375 | 0,37549 | |||
| 1918 | 0,07700 | 0,17800 | 0,34900 | ||
| 1919 | 0,08211 | 0,19076 | 0,36088 | ||
| 1920 | 0,08299 | 0,18961 | 0,34922 | ||
| 1921 | 0,08826 | 0,19154 | 0,34373 | ||
| 1922 | 0,08726 | 0,19032 | 0,34437 | ||
| 1923 | 0,08894 | 0,19344 | 0,35341 | ||
| 1924 | 0,09086 | 0,19781 | 0,36226 | ||
| 1925 | 0,09529 | 0,20310 | |||
| 1926 | 0,09369 | 0,19252 | |||
| 1927 | 0,09606 | 0,19336 | |||
| 1928 | 0,04504 | 0,09808 | 0,19065 | ||
| 1929 | 0,04926 | 0,10151 | 0,18991 | ||
| 1930 | 0,04934 | 0,10520 | 0,18880 | ||
| 1931 | 0,05520 | 0,11268 | 0,19229 | ||
| 1932 | 0,05192 | 0,10747 | 0,18327 | ||
| 1933 | 0,05622 | 0,11335 | 0,20057 | ||
| 1934 | 0,05710 | 0,11467 | 0,21236 | ||
| 1935 | 0,05546 | 0,10840 | |||
| 1936 | 0,05729 | 0,10688 | |||
| 1937 | 0,05465 | 0,09790 | |||
| 1938 | 0,02959 | 0,05827 | 0,10017 | ||
| 1939 | 0,03010 | 0,06052 | 0,09924 | ||
| 1940 | 0,03302 | 0,06352 | 0,09905 | ||
| 1941 | 0,03092 | 0,06018 | 0,09234 | ||
| 1942 | 0,03575 | 0,07190 | 0,10703 | ||
| 1943 | 0,03578 | 0,06781 | 0,10991 | ||
| 1944 | 0,03362 | 0,06568 | 0,12160 | ||
| 1945 | 0,03354 | 0,06331 | |||
| 1946 | 0,02993 | 0,05383 | |||
| 1947 | 0,03161 | 0,05289 | |||
| 1948 | 0,03427 | 0,05342 | |||
| 1949 | 0,03453 | 0,05083 | |||
| 1950 | 0,03654 | 0,05103 | |||
| 1951 | 0,03739 | 0,04966 | |||
| 1952 | 0,03740 | 0,05016 | |||
| 1953 | 0,03818 | 0,05477 | |||
| 1954 | 0,03718 | 0,05791 | |||
| 1955 | 0,03717 | ||||
| 1956 | 0,03541 | ||||
| 1957 | 0,03315 | ||||
| 1958 | 0,03122 | ||||
| 1959 | 0,03028 | ||||
| 1960 | 0,03009 |
| Year of birth cohort | q 20-30 | q 30-40 | q 40-50 | q 50-60 | q 60-70 |
| 1900 | 0,15692 | ||||
| 1901 | 0,16500 | ||||
| 1902 | 0,16042 | ||||
| 1903 | 0,16641 | ||||
| 1904 | 0,16480 | ||||
| 1905 | 0,16690 | ||||
| 1906 | 0,16734 | ||||
| 1907 | 0,16942 | ||||
| 1908 | 0,06422 | 0,16624 | |||
| 1909 | 0,06936 | 0,16961 | |||
| 1910 | 0,06459 | 0,16901 | |||
| 1911 | 0,07286 | 0,17218 | |||
| 1912 | 0,06382 | 0,16082 | |||
| 1913 | 0,07024 | 0,17688 | |||
| 1914 | 0,06335 | 0,16346 | |||
| 1915 | 0,07193 | 0,18013 | |||
| 1916 | 0,07298 | 0,17782 | |||
| 1917 | 0,08247 | 0,19099 | |||
| 1918 | 0,03296 | 0,07409 | 0,17613 | ||
| 1919 | 0,03554 | 0,07913 | 0,18403 | ||
| 1920 | 0,03466 | 0,07828 | 0,18003 | ||
| 1921 | 0,03623 | 0,07891 | 0,18248 | ||
| 1922 | 0,03301 | 0,07524 | 0,17532 | ||
| 1923 | 0,03259 | 0,07552 | 0,17849 | ||
| 1924 | 0,03256 | 0,07894 | 0,18114 | ||
| 1925 | 0,03342 | 0,07953 | |||
| 1926 | 0,03307 | 0,07642 | |||
| 1927 | 0,03414 | 0,07718 | |||
| 1928 | 0,01622 | 0,03330 | 0,07351 | ||
| 1929 | 0,01725 | 0,03496 | 0,07432 | ||
| 1930 | 0,01660 | 0,03438 | 0,07172 | ||
| 1931 | 0,01832 | 0,03765 | 0,07612 | ||
| 1932 | 0,01626 | 0,03472 | 0,07010 | ||
| 1933 | 0,01756 | 0,03748 | 0,07823 | ||
| 1934 | 0,01724 | 0,03801 | 0,08256 | ||
| 1935 | 0,01661 | 0,03582 | |||
| 1936 | 0,01668 | 0,03476 | |||
| 1937 | 0,01562 | 0,03175 | |||
| 1938 | 0,01036 | 0,01605 | 0,03189 | ||
| 1939 | 0,01056 | 0,01623 | 0,03148 | ||
| 1940 | 0,01088 | 0,01758 | 0,03212 | ||
| 1941 | 0,00974 | 0,01636 | 0,02970 | ||
| 1942 | 0,01091 | 0,01967 | 0,03501 | ||
| 1943 | 0,01108 | 0,01954 | 0,03657 | ||
| 1944 | 0,01021 | 0,01859 | 0,04022 | ||
| 1945 | 0,00985 | 0,01808 | |||
| 1946 | 0,00847 | 0,01553 | |||
| 1947 | 0,00869 | 0,01532 | |||
| 1948 | 0,00901 | 0,01517 | |||
| 1949 | 0,00875 | 0,01443 | |||
| 1950 | 0,00910 | 0,01457 | |||
| 1951 | 0,00911 | 0,01411 | |||
| 1952 | 0,00886 | 0,01411 | |||
| 1953 | 0,00880 | 0,01524 | |||
| 1954 | 0,00859 | 0,01578 | |||
| 1955 | 0,00868 | ||||
| 1956 | 0,00820 | ||||
| 1957 | 0,00792 | ||||
| 1958 | 0,00776 | ||||
| 1959 | 0,00773 | ||||
| 1960 | 0,00743 |